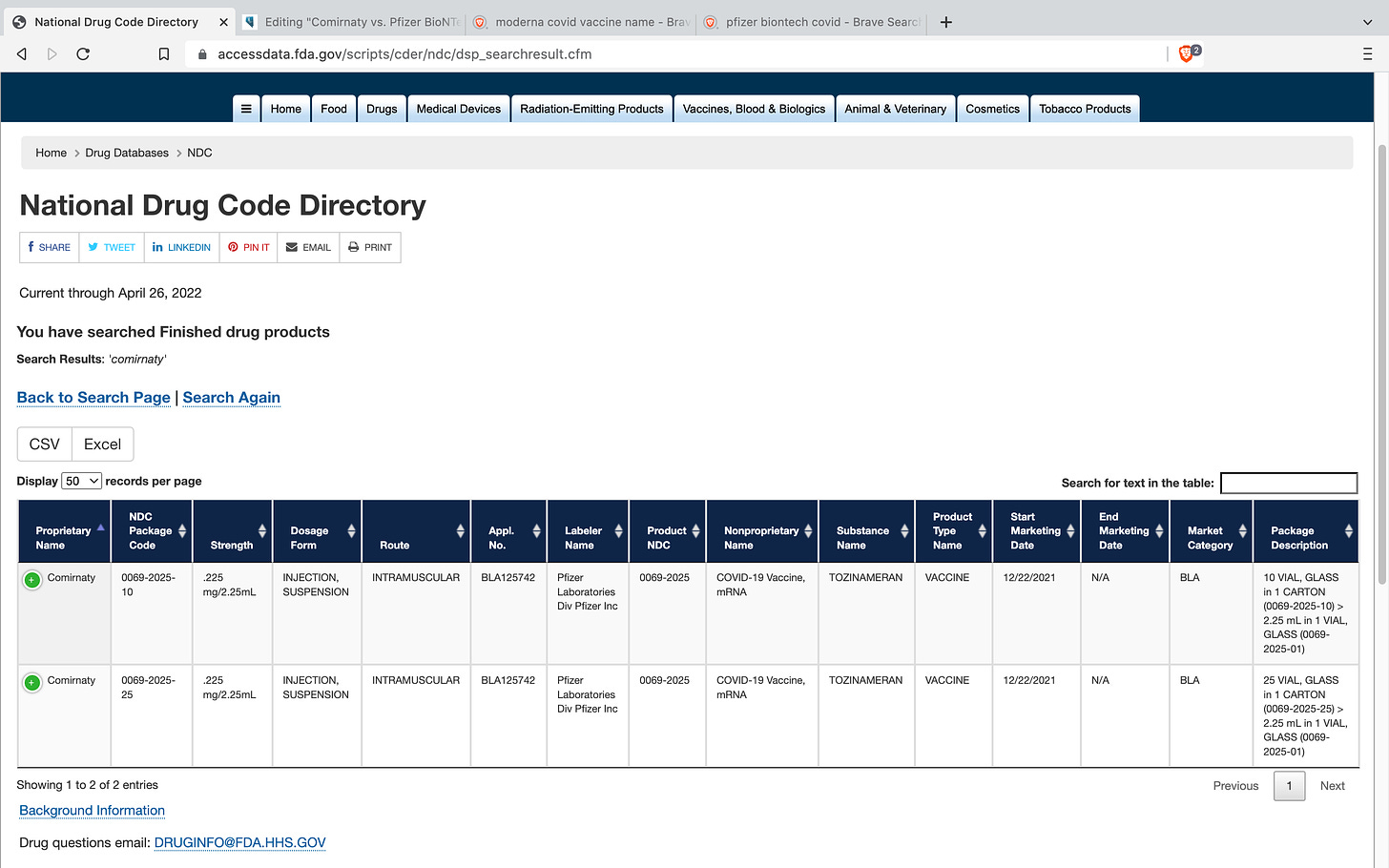
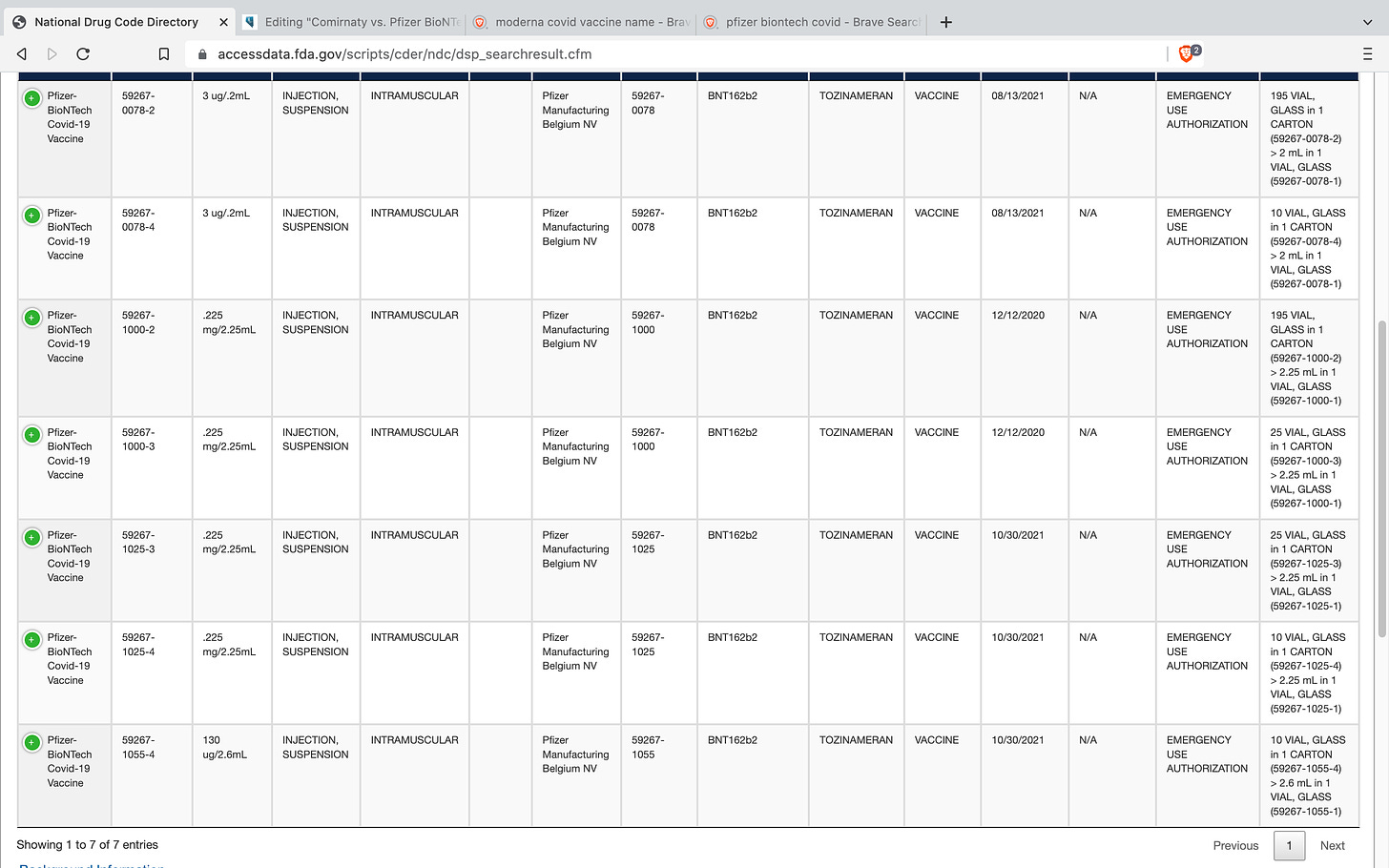
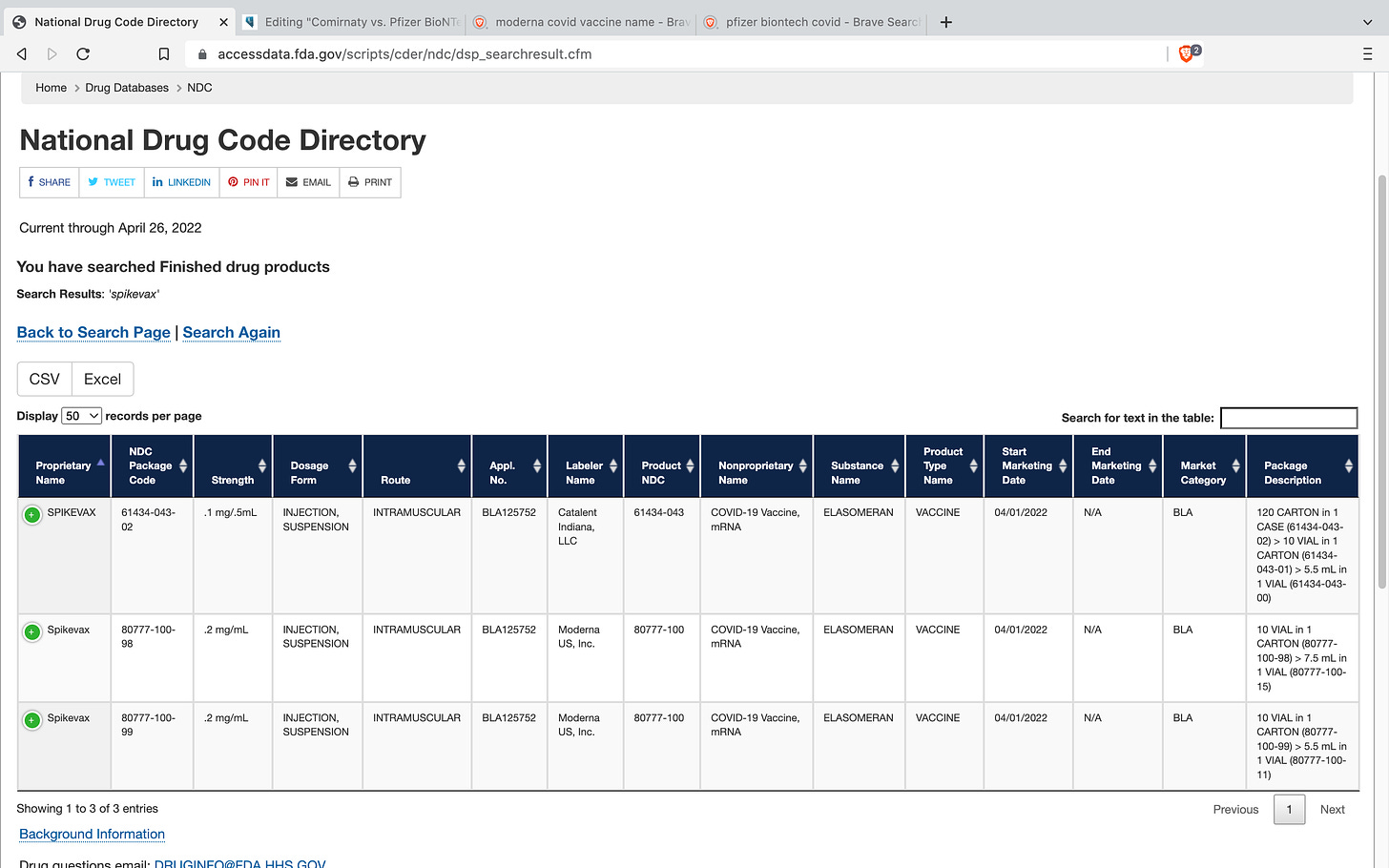
The FDA assigns a unique National Drug Code (NDC) to each and every drug and these codes are generated by the original application that the manufacturer submits to the FDA prior to beginning any of their trials. According to the FDA website, the NDC database is updated daily. Most of us know that both Pfizer and Moderna have been playing a shell game with their covid-19 vaccine products, and this is proof of that. Comirnaty and Pfizer Biontech have different NDC codes. Spikevax and Moderna have different NDC codes.
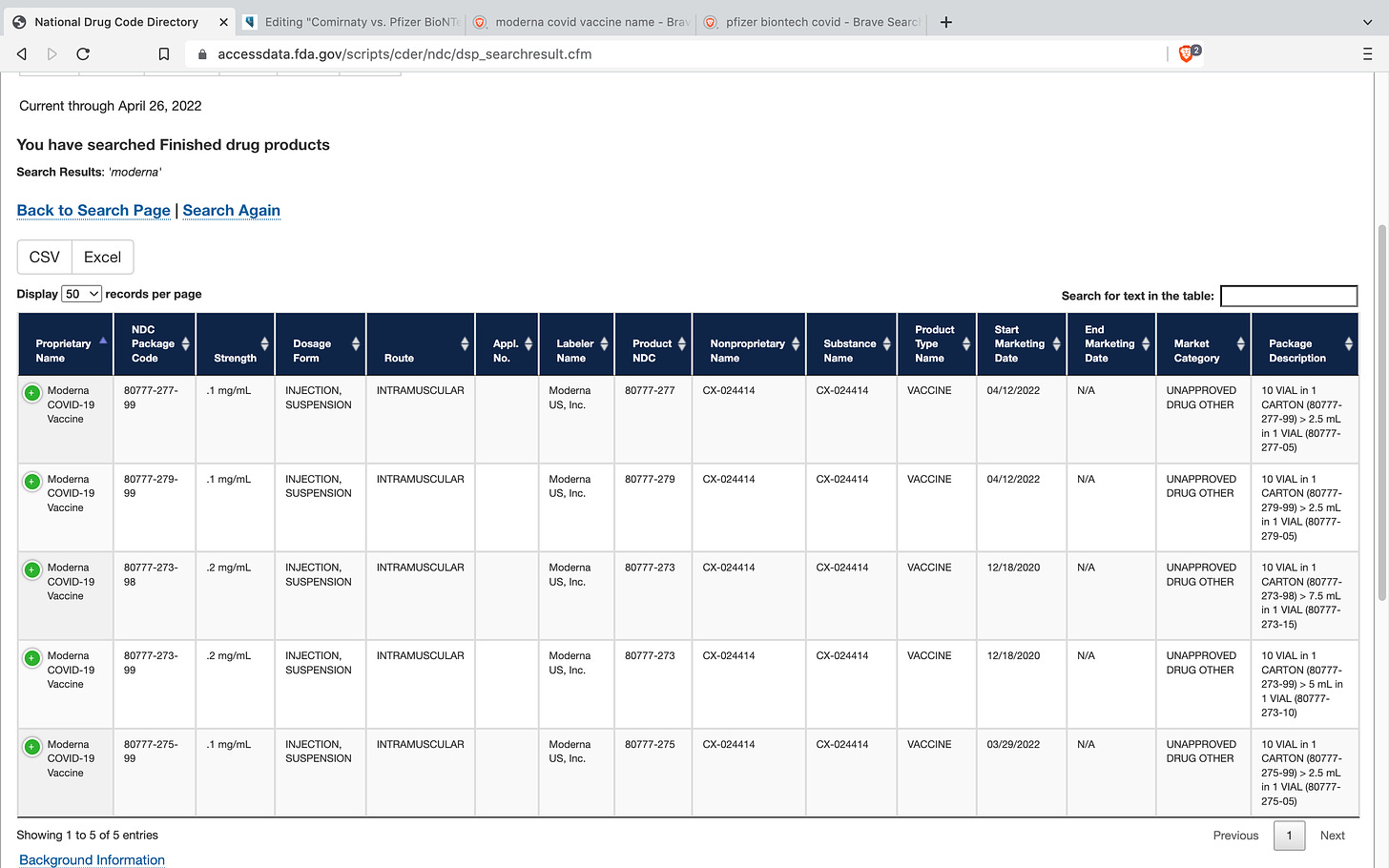
These screen shots are from this week - 4/26/22. It is interesting to note that the Moderna Covid Vaccine, which was under EUA, is now listed as an unapproved drug. I don’t recall seeing anything about the EUA on this having expired so I’ll do some research into this as soon as I have the time and write another article about it. If anyone knows anything please share that with me in the comments below. I’m also curious to know whether or not the Moderna shot is being administered currently in the United States. Because it sure seems as though it couldn’t be given its current regulatory “approval” status.
Here are the products and their current status per the NDC database.
Comirnaty (Tozinameran) - NDC# 0069-2025 FDA Approved Market Category BLA (Biologics License Application)
https://www.accessdata.fda.gov/scripts/cder/ndc/dsp_searchresult.cfm
Pfizer BioNTech Covid-19 Vaccine (BNT162b2 or Tozinerameran) - NDC #59267-078, #59267-1000, #59267-1025 and #59267-1055 all under FDA Emergency Use Authorization (EUA)
https://www.accessdata.fda.gov/scripts/cder/ndc/dsp_searchresult.cfm
SpikeVax (Elasomeran) - NDC#61434-043 and #80777-100 FDA Approved Market Category BLA
https://www.accessdata.fda.gov/scripts/cder/ndc/dsp_searchresult.cfm
Moderna Covid-19 Vaccine (CX-024414) - NDC#80777-277, #80777-279, #80777-273, #80777-275 all in FDA Market Category Unapproved Drug Other
https://www.accessdata.fda.gov/scripts/cder/ndc/dsp_searchresult.cfm
So what happened to the EUA for Moderna? It looks like we have a new player in the game. Novovax’s brand new injection is showing up in the NDC Market Category as Emergency Use Authorized. Preliminary research into this product shows that it was developed at the University of Washington under the name of GPB510 and licensed by a company called SK biosciences. A company and a project that BMG, CEPI and GAVI have their hands ALL OVER. Given that Moderna is also a Gates product, might we assume that this new one has hit the market simply to replace his old one?
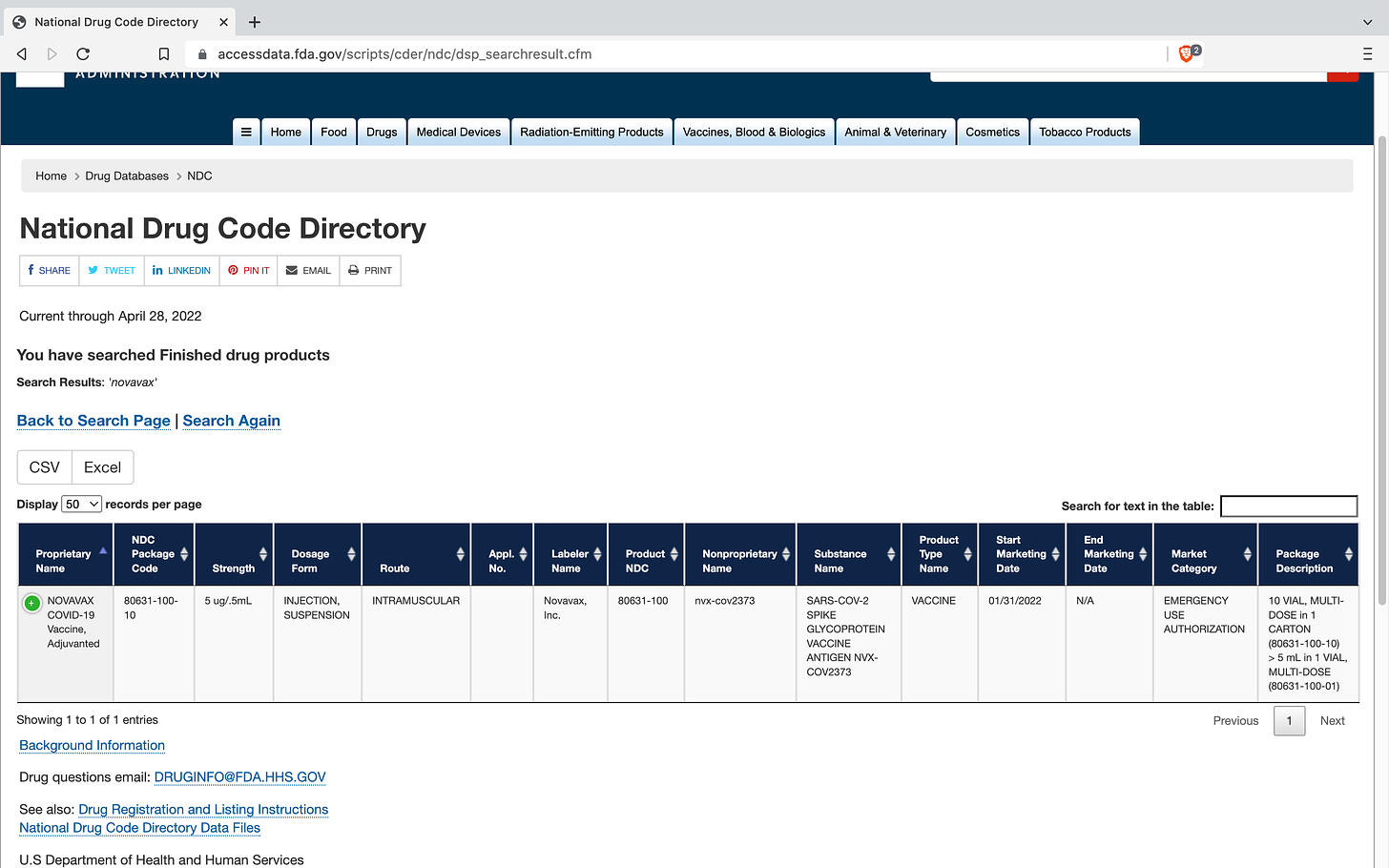
Novavax Covid-19 Vaccine Adjunvanted (nvx-cov2373) - NDC#80631-100; Market Category FDA Emergency Use Authorization
https://www.accessdata.fda.gov/scripts/cder/ndc/dsp_searchresult.cfm
Needless to say, the liability con game continues. Please stop referring to these shots by their “umbrella” names of Pfizer and Moderna and use the specific name for the shot. Those under EUA are completely liability-free for the manufacturer and the government. Once an approved product has been added to the CDC’s list of Recommended Childhood Vaccines, it is completely liability free for the manufacturer and for the government. (See my previous article about this topic HERE) The general public needs to become aware of the difference and what that means to them should they or a loved one experience an injury….or worse. Because the way this has all been set up prevents them from obtaining ANY financial support from ANYONE in the event of future medical issues and related expenses. Just ask any parent of a vaccine injured child about who it is that’s left “holding the ball”.